Abstract
The prognosis of acute myeloid leukemia (AML) is very poor, with overall survival rates of approximately 50% in children and 25% in adults, despite reaching the limits of conventional cytotoxic chemotherapy. Therapeutic targeting in AML through either surface antigens or aberrantly activated cell signaling pathways are two emerging targeted treatment strategies in efforts to further improve outcomes. FLT3/ITD mutations are among the most common somatic mutations in AML and confer an inferior prognosis. Outcomes for this group of patients have recently been shown to be improved with targeting mutant FLT3 with FLT3 inhibitors (FLT3i). Patients with FLT3/ITD mutant AML express high levels of CD33, and the benefit of CD33 targeted agents in this group of patients has been demonstrated. Thus, we sought to investigate the combination strategy of FLT3i with SGN-CD33A (vadastuximab tarline), a CD33-directed antibody drug conjugate (ADC), in FLT3/ITD AML.
Combination experiments with 6 FLT3 inhibitors (midostaurin, quizartinib, crenolanib, gilteritinib, AMG925, G-749) and SGN-CD33A were performed in 2 FLT3/ITD positive cell lines (MV4-11, MOLM-13). Five non-FLT3 mutant cell lines (Kasumi1, KG-1, ME-1, TF1, THP1) as well as normal hematopoietic cells (CD34+ HSCs, megakaryocytes) with and without serum were used as controls. In each pairing, a total of 240 individual drug combinations of SGN-CD33A and FLT3i were evaluated at doses spanning 4 and 6 log-folds respectively. Across the matrix of dose combinations, we compared observed cytotoxicity against 3 standard additivity models (Loewe, Bliss, and Highest Single Agent (HSA)) and summarized each experiment using metrics gauging the degree of synergy or antagonism observed based on those additive models. The combination was also evaluated with xenograft experiments. Following transplantation with MOLM-13 cells, mice received no treatment or treatment with FLT3i, SGN-CD33A, or the combination and survival was evaluated using Kaplan-Meier methods.
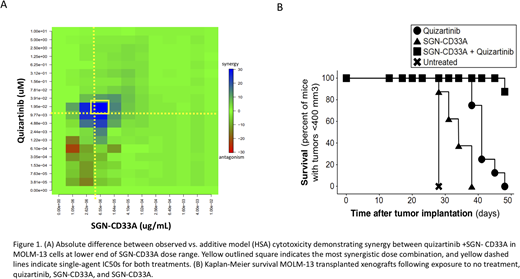
We performed a total of 88 in vitro cell line experiments, 25 in FLT3/ITD positive cell lines. Aggregate statistics over all dose combinations showed significant synergistic effects of the SGN-CD33A/FLT3i combinations in FLT3/ITD mutant cells. Importantly, no synergistic effects were observed in any of the non-mutant cell lines. Moreover, in most of the experiments in FLT3/ITD-positive cell lines the dose combination producing the highest synergistic effect was within one log of the IC50 for both SGN-CD33A and the FLT3i (Fig 1A). These findings indicate that synergy was achieved at clinically meaningful doses. We subsequently evaluated the effect of the combination in vivo and following transplantation of MOLM-13 cells, xenografts were treated with either quizartinib alone, SGN-CD33A alone, quizartinib+SGN-CD33A, or no treatment. Subjects treated with SGN-CD33A/FLT3i combination had improved survival compared to the untreated or single agent mice (Figure 1B).
Patients with FLT3/ITD mutations have a poor prognosis, despite intensive therapy including hematopoietic stem cell transplant. Importantly, this group of patients harbors 2 potential therapeutic targets, namely aberrant FLT3 activation and high CD33 surface expression. We have shown combining FLT3 inhibitors with the CD33 directed ADC SGN-CD33A results in a synergistic effect with increased cell killing and prolonged survival in xenografts. This synergistic effect was most pronounced at doses on the lower range of SGN-CD33A, suggesting that a lower dose of CD33-directed ADCs could be used thus mitigating excess hematopoietic toxicity. These treatments have little overlap in toxicity profiles suggesting that they can be safely combined in patients, and further clinical investigation of this strategy is warranted.
Thurman:Seattle Genetics: Employment, Equity Ownership. Rohm:Seattle Genetics: Employment, Equity Ownership. Biechele:Seattle Genetics: Employment, Equity Ownership. Arthur:Seattle Genetics: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal